Abstract
Background. We report here a trial in progress for the evaluation of a novel system aimed to provide an all-digital standardized bone marrow aspirate (BMA) analysis, Scopio Labs X100, empowered by artificial intelligence (AI) based cell pre-classification. Current methods for the analysis and reporting of BMA specimens are based on analog microscopy, as whole slide imaging at x100 magnification is not practically available. The lack of uniformity between experts in the field, originating from a subjective manual review, can lead to inconsistencies in disease diagnosis and classification, and thereby affect treatment and clinical outcomes. For example, ICSH and WHO guidelines require that at least 500 cells should be counted in at least two smears when a precise percentage of an abnormal cell type is required for diagnosis and classification. It is also recommended that in order reduce imprecision from sampling error, the total number of cells counted in the differential should be increased, specifically if the abnormal cell count is very close to a critical threshold for disease stratification or response assessment. For the general evaluation of hematopoiesis, Myeloid to Erythroid (M:E) ratio is reported. Considering the complexity of the manual BMA analysis, even more so in routine laboratory settings with competitive turnaround times, a digital transformation can sustain the desired standardization, and increase sensitivity and efficiency in routine workflow.
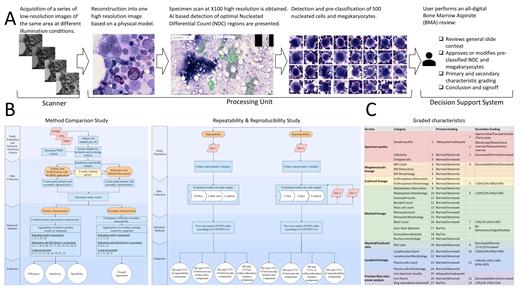
Study Design and Methods. This multisite study is taking place at: Hospital of the University of Pennsylvania (HUP), Oregon Health and Science University (OHSU), and Tel Aviv Sourasky Medical Center (TASMC). BMA analysis is performed with a manual microscope as the reference arm and in Scopio Labs X100 Full Field BMA application as the test arm (Figure 1A). Two hematopathologists at each site independently review 265 BMA specimens, including 205 with a Romanowsky stain and 60 with a Prussian Blue stain, in both the test and the reference arms. There is a 3 week washout period between arms (Figure 1B, right). ICSH guidelines were rigorously translated into a comprehensive report format used in both study arms. The report presents 27 primary and 13 secondary characteristics for the morphological assessment of BMA (Figure 1C). These include evaluation of specimen quality, evaluation of count, maturation and morphology of trilineage hematopoietic elements (myeloid, erythroid and megakaryocytic), as well as lymphocytes and plasma cells. For a repeatability study, 8 representative samples are analyzed through 20 days, 2 daily runs and 2 replicas in one site. For reproducibility study, 8 representative samples are analyzed in all sites for 5 days with 5 replicas (Figure 1B, left).
The collected BMA samples hold a distribution of 55.61% males, with 2.02%, 9.46%, 16.39%, 54.73% and 17.40% of ages 13-21, 22-39, 40-55, 56-75 and >75 respectively. All samples were diagnosed by WHO criteria. Diagnoses include AML, ALL, MPN, MDS, PCN, lymphoid neoplasms, aplastic anemia, ITP and normal morphology marrow and hemodiluted samples. All samples were retrieved from the sites' bone marrow sample storage.
For the method comparison study, the primary and secondary characteristics are aggregated into three primary and secondary evaluation categories of specimen quality, count, and morphology and maturation assessments (Figure 1B, left, 1C). For the primary groups, confusion matrix will be produced. For the secondary groups, contingency tables will be generated (Figure 1B, left). For the repeatability and reproducibility (R&R) studies, two-way nested ANOVA tables will be created (Figure 1, right). Primary groups will be measured for accuracy in the form of efficiency, sensitivity and specificity. Secondary groups will be measured for overall agreement. R&R will be measured for SD and CV.
The introduction of Scopio's full field morphological evaluation of BMA smears, promotes an accurate diagnosis of hematological disorders including hematological malignancies, and enables a remote evaluation of BMA smears. By reviewing the entire BMA smear, and by counting a very large number of cells, this novel approach provides a new and highly accurate tool for early detection of pathological conditions, including residual disease following therapy.
Bagg: Scopio Labs: Research Funding. Raess: Scopio Labs: Research Funding. Jengehino: Scorpio Labs: Other: Partial Salary Support. Wiszniewska: Scopio Labs: Research Funding. Huynh: Scorpio Labs: Other: Salary Support. Fan: Scopio Labs: Research Funding. Bhattacharyya: Scorpio Labs: Other: Partial Salary Support. Avivi: Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau. Katz: Scopio Labs: Consultancy.